Quality
Process, Quality and Supplier Management
Quality for your success
In order for you to be successful, MAGNESIA stands for personal service and also for the best standard. We ensure this by establishing a comprehensive quality, process and supplier management at the highest level throughout the company.
Certification
MAGNESIA is GDP, ISO, HACCP, IFS Logistics and IFS Broker certified. The quality of all processes is ensured by our ISO9001-based quality management system, which we have implemented in 1995 and since then continuously improved and annually audited by an external certification organization. Likewise, our HACCP system for ensuring product quality is also audited for the use of the products we supply for food production.
At regular intervals, Magnesia is inspected by the regulatory body for trade in active pharmaceutical ingredients (GDP). We have been audited on the basis of the International Food Standards (IFS) since 2023. In addition, we are registered as a wholesaler of feed for pets and farm animals with the Lower Saxony State Office for Consumer Protection and Food Safety.
Product quality
Our products always guarantee both, the highest possible quality and ideal characteristics. Our wide range of raw materials allows us to work with you to identify products that meet your requirements and ensure their timely delivery on-site.
Pharmaceutical quality
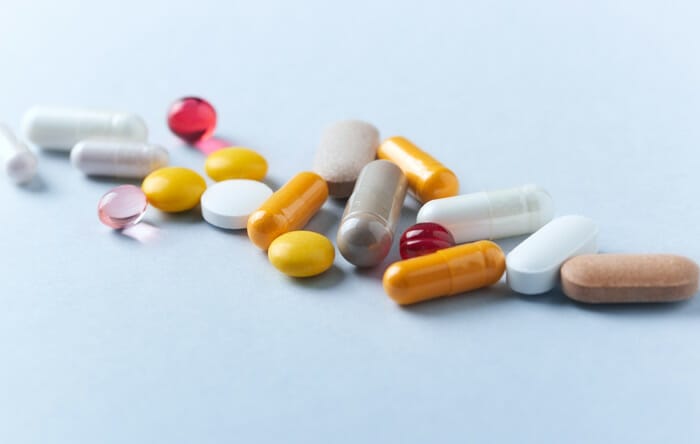
Our mineral compounds in pharmaceutical grade comply with the strict specifications of the currently valid pharmacopoeias (EP, BP, USP, etc.). Raw materials of this class can be used directly as additives for drugs. For specific products, there is also a qualification for use as an active ingredient in drugs.
Food quality

Mineral compounds, for use in the production of food, meet the relevant quality and distribution requirements for this industry. Where available and required, the specifications meet the requirements e.g. for food additives, or of the FCC.
Technical quality

Magnesium and calcium compounds are indispensable as raw and auxiliary materials in many manufacturing processes. We supply the processing industry with numerous products in technical quality.
Aviation & Aerospace quality

For special industrial requirements, we supply chemical compounds in the required purity and weight grades. Properties such as bulk density or chemical activity are precisely specified.
Supplier qualification as an integrated process
As part of the qualification process of our suppliers, we carry out appropriate audits and ensure that requirements are met. Even after successful qualification, compliance with legal requirements is regularly assessed. Our integrated process ensures that all changes at supplier facilities or to the product that could affect manufacturing or test parameters are checked and approved before implementation.